what phylum does plasmodium belong to? (use your textbook.)
Full general Concepts
Clinical Manifestations
Initially patients have fever, chills, sweating, headache, weakness, and other symptoms mimicking a "viral syndrome." Subsequently, astringent disease may develop, with an aberrant level of consciousness, severe anemia, renal failure, and multisystem failure.
Nomenclature
Plasmodia are protozoa. Only the species Plasmodium falciparum, P vivax, P malariae, and P ovale are usually infectious for humans. Of these, P falciparum is the nearly dangerous.
Structure and Life Cycle
In nature, uninucleate sporozoites in the salivary glands of infected mosquitoes are injected into a homo host when the mosquito feeds. The sporozoites chop-chop invade liver parenchymal cells, where they mature into liver-stage schizonts, which flare-up to release 2,000 to 40,000 uninucleate merozoites. In P vivax and P ovale infections, maturation of the schizont may be delayed for 1 to 2 years. Each merozoite can infect a reddish blood cell. Inside the red jail cell, the merozoite matures either into a uninucleate gametocyte--the sexual stage, infectious for Anopheles mosquitoes--or, over 48 to 72 hours, into an erythrocyticstage schizont containing x to 36 merozoites. Rupture of the schizont releases these merozoites, which infect other red cells. If a vector mosquito ingests gametocytes, the gametocytes develop in the musquito gut to gametes, which undergo fertilization and mature in 2 to 3 weeks to sporozoites.
Pathogenesis
The fever and chills of malaria are associated with the rupture of erythrocytic-stage schizonts. In severe falciparum malaria, parasitized red cells may obstruct capillaries and postcapillary venules, leading to local hypoxia and the release of toxic cellular products. Obstacle of the microcirculation in the brain (cerebral malaria) and in other vital organs is thought to be responsible for astringent complications. Cytokines (e.one thousand., tumor necrosis factor) are likewise felt to be involved, simply at present their role is unclear.
Host Defenses
Both innate and acquired immunity occur. Innate amnesty consists of diverse traits of erythrocytes that discourage infection. The sickle-jail cell trait protects against the development of astringent P falciparum malaria, and the absenteeism of Duffy antigen prevents infection by P vivax. Recurrent infections lead to the development of humoral and cellular immune responses against all Plasmodium stages. Acquired amnesty does not prevent reinfection but does reduce the severity of disease.
Epidemiology
Malaria is distributed worldwide throughout the tropics and subtropics.
Diagnosis
Diagnosis depends primarily on the identification of plasmodia in thick and thin blood smears.
Control
Treatment: The widespread resistance of P falciparum to chloroquine complicates treatment of falciparum malaria. Alternative drugs such as mefloquine, pyrimethamine/ sulfadoxine (FansidarR), quinine, quinidine, halofantrine and artemisinin derivatives (qinghaosu) are used. Chloroquine remains highly effective against P malariae and P ovale malaria, and against P vivax everywhere except Papua New Guinea and parts of Indonesia, where significant resistance has developed. Affliction acquired by P vivax and P ovale requires primaquine to eradicate latent liver forms of the parasite.
Prevention: Malaria may exist prevented by chemoprophylaxis and personal protective measures confronting the mosquito vector and by community-wide measures to control the vector. Exposure to night-feeding Anopheles mosquitoes is reduced by using protective vesture, insect repellents, insecticides, insecticide-impregnated bed nets, etc. Mosquitoes may be reduced past destroying breeding places and by application of insecticides. Vaccines are being developed.
Introduction
Malaria has been a major disease of humankind for thousands of years. It is referred to in numerous biblical passages and in the writings of Hippocrates. Although drugs are available for treatment, malaria is still considered by many to be the nearly important infectious disease of humans: there are approximately 200 million to 500 million new cases each year in the world, and the illness is the direct cause of i million to two.5 1000000 deaths per twelvemonth.
Malaria is caused by protozoa of the genus Plasmodium. Four species cause disease in humans: P falciparum, P vivax, P ovale and P malariae. Other species of plasmodia infect reptiles, birds and other mammals. Malaria is spread to humans by the bite of female mosquitoes of the genus Anopheles.
Clinical Manifestations
The most characteristic symptom of malaria is fever. Other common symptoms include chills, headache, myalgias, nausea, and vomiting. Diarrhea, intestinal hurting, and cough are occasionally seen. As the illness progresses, some patients may develop the classic malaria paroxysm with bouts of illness alternate with symptom-free periods (Fig. 83-1). The malaria paroxysm comprises three successive stages. The first is a xv-to-60 minute common cold phase characterized past shivering and a feeling of cold. Next comes the 2-to-6 hour hot stage, in which there is fever, sometimes reaching 41°C, flushed, dry out pare, and often headache, nausea, and vomiting. Finally, in that location is the 2-to-4 60 minutes sweating stage during which the fever drops rapidly and the patient sweats. In all types of malaria the periodic febrile response is caused by rupture of mature schizonts. In P vivax and P ovale malaria, a breed of schizonts matures every 48 hr, so the periodicity of fever is tertian ("tertian malaria"), whereas in P malariae disease, fever occurs every 72 hours ("quartan malaria"). The fever in falciparum malaria may occur every 48 60 minutes, but is usually irregular, showing no distinct periodicity. These classic fever patterns are usually non seen early in the form of malaria, and therefore the absence of periodic, synchronized fevers does not rule out a diagnosis of malaria.

Effigy 83-i
Typical temperature charts of malarial infections. (Adapted from Bruce-Chwatt LJ: Essential Malariology. 2d Ed. John Wiley and Sons, New York, 1985, p. 52, with permission.)
Physical findings in malaria are nonspecific and offering little help in diagnosis. In many cases there may be no positive findings other than fever. Splenomegaly is common just may not be apparent early in illness. Hepatomegaly, jaundice, hypotension and abdominal tenderness may too be seen. Malaria does not cause lymphadenopathy and is not associated with a rash.
A variety of laboratory abnormalities may be seen in a case of unproblematic malaria. These include normochromic, normocytic anemia, thrombocytopenia, leukocytosis or leukopenia, hypoglycemia, hyponatremia, elevated liver and renal function tests, proteinuria, and laboratory testify of disseminated intravascular coagulation (although clinically important bleeding is rare). Eosinophilia is non seen. Patients with complicated malaria occasionally show evidence of massive intravascular hemolysis with hemoglobinemia and hemoglobinuria.
If the diagnosis of malaria is missed or delayed, peculiarly with P falciparum infection, potentially fatal complicated malaria may develop. The well-nigh frequent and serious complications of malaria are cerebral malaria and severe anemia. Cerebral malaria is divers as whatsoever abnormality of mental status in a person with malaria and has a instance fatality rate of 15 to 50 pct. Other complications include: hyperparasitemia (more than three to 5 percent of the erythrocytes parasitized); astringent hypoglycemia; lactic acidosis; prolonged hyperthermia; stupor; pulmonary, cardiac, hepatic, or renal dysfunction; seizures; spontaneous bleeding; or high-output diarrhea or vomiting. These manifestations are associated with poor prognosis. Persons at increased risk of severe affliction from malaria include older persons, children, pregnant women, nonimmune persons and those with underlying chronic illness. Other complications of malaria infection include gram-negative sepsis, aspiration pneumonia and splenic rupture.
Classification
Only iv species of the protozoan genus Plasmodium unremarkably infect humans: P falciparum, P vivax, P malariae, and P ovale (Fig. 83-2). P falciparum and P vivax account for the vast majority of cases. P falciparum causes the near severe illness.

Figure 83-2
Blood stages of Plasmodium. Column A, Plasmodium vivax; B, P ovale; C, P malariae; D, P falciparum. Row 1, young trophozoites (band forms); 2, growing trophozoites; 3, mature trophozoites; 4, mature schizonts; 5, macrogametocytes; 6, microgametocytes. (more...)
Construction and Life Cycle
Like many protozoa, plasmodia pass through a number of stages in the form of their 2-host life cycle. The stage infective for humans is the uninucleate, lancet-shaped sporozoite (approximately ane × 7 μm). Sporozoites are produced by sexual reproduction in the midgut of vector anopheline mosquitoes and migrate to the salivary gland. When an infected Anopheles mosquito bites a human, she may inject sporozoites along with saliva into small claret vessels (Fig. 83-three). Sporozoites are thought to enter liver parenchymal cells within xxx minutes of inoculation. In the liver cell, the parasite develops into a spherical, multinucleate liver-phase schizont which contains two,000 to twoscore,000 uninucleate merozoites. This process of enormous distension is called exoerythrocytic schizogony. This exoerythrocytic or liver phase of the disease usually takes betwixt 5 and 21 days, depending on the species of plasmodium. However, in P vivax and P ovale infections, maturation of liver-stage schizonts may be delayed for equally long as 1 to ii years. These quiescent liver-stage parasites are called hypnozoites.
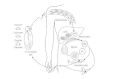
Figure 83-3
Life wheel of malaria parasite. (Adapted from Miller LH, Howard RJ, Carter R et al: Inquiry toward malaria vaccines. Science 234:1350, 1986, with permission.)
Regardless of the time required for development, the mature schizonts somewhen rupture, releasing thousands of uninucleate merozoites into the bloodstream. Each merozoite can infect a red blood prison cell. Within the red cell, the merozoite develops to grade either an erythrocytic-stage (blood-stage) schizont (by the procedure of erythrocytic schizogony) or a spherical or banana-shaped, uninucleate gametocyte. The mature erythrocytic-stage schizont contains 8 to 36 merozoites, each 5 to 10 μm long, which are released into the blood when the schizont ruptures. These merozoites proceed to infect another generation of erythrocytes. The fourth dimension required for erythrocytic schizogony-which determines the interval betwixt the release of successive generations of merozoites-varies with the species of plasmodium and is responsible for the classic periodicity of fever in malaria (Fig 83-1).
The gametocyte, which is the sexual stage of the plasmodium, is infectious for mosquitoes that ingest it while feeding. Within the musquito, gametocytes develop into female and male gametes (macrogametes and microgametes, respectively), which undergo fertilization and then develop over 2 to 3 weeks into sporozoites that can infect humans. The delay between infection of a mosquito and maturation of sporozoites ways that female mosquitoes mustlive a minimum of two to 3 weeks to be able to transmit malaria. This fact is important in malaria control efforts.
Pathogenesis
Clinical illness is caused by the erythrocytic stage of the parasite. No disease is associated with sporozoites, the developing liver stage of the parasite, the merozoites released from the liver, or gametocytes.
The first symptoms and signs of malaria are associated with the rupture of erythrocytes when erythrocytic-stage schizonts mature. This release of parasite cloth presumably triggers a host immune response. The cytokines, reactive oxygen intermediates, and other cellular products released during the immune response play a prominent office in pathogenesis, and are probably responsible for the fever, chills, sweats, weakness, and other systemic symptoms associated with malaria. In the case of falciparum malaria (the form that causes most deaths), infected erythrocytes adhere to the endothelium of capillaries and postcapillary venules, leading to obstruction of the microcirculation and local tissue anoxia. In the brain this causes cerebral malaria (Fig. 83-4); in the kidneys information technology may crusade astute tubular necrosis and renal failure; and in the intestines it can cause ischemia and ulceration, leading to gastrointestinal bleeding and to bacteremia secondary to the entry of intestinal bacteria into the systemic circulation. The severity of malaria-associated anemia tends to be related to the caste of parasitemia. The pathogenesis of this anemia appears to exist multifactorial. Hemolysis or phagocytosis of parasitized erythrocytes and ineffective erythropoiesis are the most important factors, and phagocytosis of uninfected erythrocytes and an autoimmune hemolytic anemia have as well been implicated. Massive intravascular hemolysis leading to hemoglobinuria and renal failure is referred to equally blackwater fever. It was described more than frequently in the by than currently. Hemolysis may also occur after the utilize of certain antimalarials (specially primaquine) in patients with glucose 6-phosphate dehydrogenase deficiency.

Figure 83-4
Light micrograph of a cognitive capillary blocked with parasitized erythrocytes. This specimen is from a patient with cerebral malaria. (From Aikawa M: Morphological changes in erythrocytes induced by malarial parasites. Biol Prison cell 64:174, 1988, with permission.) (more than...)
Host Defenses
Susceptibility to malaria infection and disease is regulated by hereditary and acquired factors (Fig 83-5). It now seems clear that the sickle prison cell trait (which is the cause of sickle-jail cell anemia) developed as a balanced polymorphism to protect against serious P falciparum disease. Although individuals with sickle prison cell anemia or the sickle cell trait are as easily infected with malaria parasites as normal individuals, they rarely showroom malaria disease considering P falciparum develops poorly in their erythrocytes. The virtual absence of P vivax infections in many areas of Africa is explained by the fact that well-nigh blacks do non have Duffy blood-group antigens, which apparently part as erythrocyte surface receptors for P vivax merozoites; without the Duffy antigen, the parasites cannot invade. Malaria parasites do non develop well in ovalocytes, and it has been suggested that ovalocytosis, which is quite mutual in some malarious areas, such equally New Guinea, may reduce the incidence of malaria. Some investigators accept suggested that glucose 6-phosphate dehydrogenase deficiency, every bit well as a number of other hemoglobinopathies (including the thalassemias and hemoglobin E), also protect against malaria infection, but the evidence for these associations is less compelling.

Figure 83-5
Host defense against malaria. (Adjusted from Miller LH, Howard RJ, Carter R et al: Inquiry toward malaria vaccines. Science 234:1350, 1986, with permission.)
Acquired immunity can as well protect against malaria infection and the development of malaria disease. In malarious areas, both the prevalence and severity of malaria infections decrease with age. Nonetheless, in contrast to many viral infections, multiple infections with malaria exercise non confer longlasting, sterile protective immunity. Virtually all adults in malarious areas suffer repeated malaria infections. Individuals who are repeatedly exposed to malaria develop antibodies against many sporozoite, liver-stage, blood-phase, and sexual-stage malaria antigens. Information technology is idea that antibodies acting confronting sporozoites, liver-stage and blood-stage organisms are responsible for the decreased susceptibility to malaria infection and disease seen in adults in malarious areas, and that antibodies against the sexual stages of plasmodia may reduce malaria transmission. Additional work also suggests that the naturally acquired amnesty includes the release of cytokines that act against all stages of the parasite, and also a cytotoxic T prison cell response directed at liver stages of the parasite.
Caused antibody-mediated immunity is plainly transferred from mother to fetus across the placenta. This passively transferred immunity is lost inside half dozen to nine months, as is the immunity in adults if they leave a malarious area and are no longer exposed to plasmodia. Meaning women, peculiarly primigravidas, are more susceptible to malaria infections and serious illness.
Epidemiology
Malaria is transmitted primarily by the bite of infected anopheline mosquitoes. It tin as well be transmitted by inoculation of infected blood and congenitally. Anophelines feed at night and their convenance sites are primarily in rural areas. The greatest gamble of malaria is therefore from dusk to dawn in rural areas. In many malaria-endemic areas, there is little or no hazard in urban areas. However, urban manual is mutual in some parts of the globe, especially Africa.
Malaria was once transmitted in many parts of the world, for instance, equally far north equally North Dakota in the U.s.. Dueboth to environmental changes and to eradication campaigns conducted in the years subsequently Globe State of war Two, owned malaria manual has been eliminated from many areas, including the U.s.a. and Europe. The disease is still widely transmitted in the tropics and subtropics (Fig. 83-6). In these areas malaria manual may be endemic, occurring predictably every yr, or it may be epidemic, occurring sporadically when conditions are correct. Endemic transmission of malaria may be year-round or seasonal. In some areas of Africa, 90 to 100 percentage of children less than 5 years onetime take malaria parasites circulating in their blood all the time. Because naturally acquired immunity develops with increasing exposure, in endemic areas malaria disease is primarily found in children. In epidemic areas, on the other hand, naturally acquired immunity falls off between epidemics, and malaria therefore affects all historic period groups during epidemics.

Figure 83-6
Distribution of malaria and chloroquine-resistant Plasmodium falciparum, 1993. (Centers for Affliction Control and Prevention).
Approximately 1,000 cases of malaria are reported each year in the U.s. in returning travelers. Of the 1016 imported cases reported in 1991, the majority were acquired in Africa (466 cases) and India (221 cases). P vivax accounted for 43% of the cases and P. falciparum for 39%. The risk to travelers of acquiring P falciparum is greatest in Africa. This is because it is the most prevalent species in that location, malaria transmission is much more than intense there than in other parts of the world, and there is meaning risk in urban areas.
Anopheles mosquitoes capable of transmitting malaria are plant in a number of areas of the The states. Local manual may therefore occur when these mosquitoes feed upon malariainfected individuals, generally immigrants from malaria-endemic areas. Local transmission has recently occurred in southern California, New Jersey, New York Urban center, and Houston, Texas. Malaria may likewise occur when infected mosquitoes are transported into not-owned areas, such as by airplanes or ships.
In the late 1950s and early 1960s, it was thought that malaria could be eradicated through the widespread utilize of insecticides such as Dichloro-diphenyl-trichloroethane and past handling of cases with chloroquine. Eradication is no longer thought possible, still, because of the development of drug resistance by both the mosquito and the parasite, and because of deteriorating social and economic conditions in many malaria-endemic countries. These changes have resulted in a dramatic increase in the incidence of malaria in many parts of the world, and an increase in malaria-related mortality in some of these areas.
Diagnosis
In the United States, many of the deaths from malaria are the upshot of delayed diagnosis and treatment because the health care provider did not suspect malaria. The diagnosis of malaria requires a high index of suspicion; malaria should be considered in any individual who has a fever and has visited an endemic area for malaria, received a blood transfusion, or used intravenous drugs. Although 95 percent of individuals infected with malaria develop their principal illness inside 6 weeks of exposure, somemay have primary attacks up to a year after exposure, and relapses of malaria can occur up to 2–three years after exposure. Therefore, individuals having a febrile illness and a history of exposure in the last 2–3 years should be evaluated for malaria.
Definitive diagnosis of malaria generally requires direct observation of malaria parasites in Giemsa-stained thick and sparse blood smears (Fig. 83-2). Thick claret smears are more hard to interpret than sparse blood smears but they are much more sensitive, equally more than blood is examined. Sparse blood smears, in which parasites are seen inside erythrocytes, are used to determine the species of the infecting parasite. The presence of diagnostic forms can vary markedly with the phase of the life cycle, especially early in affliction. In falciparum malaria, most organisms are not present in the peripheral claret because they are sequestered in the microvascular tissue of internal organs. If malaria is suspected, blood smears should be examined every vi to 12 60 minutes for at least 2 days. New diagnostic methods include a rapid antigen-capture dipstick exam and a technique for detecting parasites with a fluorescent stain. Both of these tests are fast, easy to perform and are highly sensitive and specific
Other diagnostic methods include assays to find malaria antibodies and antigens, and polymerase concatenation reaction/DNA and RNA probe techniques. These techniques are used primarily in epidemiologic studies and immunization trials and rarely in the diagnosis of individual patients.
Command
The principles of medical direction of malaria reverberate the fact that falciparum malaria tin can progress speedily to a life-threatening state and that complications tin occur even subsequently the initiation of therapy. They are: (1) early recognition of infection due to P falciparum; (2) rapid institution of appropriate therapy; (three) recognition and therapy of complications; and (4) monitoring of clinical and parasitologic response to therapy.
Malaria therapy is complicated by the fact that parasites may be present in the claret and the liver and that different drugs are required to eradicate each. Drugs which kill malaria parasites in the claret are chosen blood-stage schizonticides and those that kill them in the liver are chosen tissue schizonticides. A clinical cure refers to the elimination of parasites from the blood, which volition save the signs and symptoms of disease. A radical cure is the eradication of all parasites from the body, both blood and liver. In cases of P falciparum and P malariae, which exercise not accept latent liver forms (hypnozoites), an effective dose of a blood schizonticide to which the parasite is sensitive should lead to radical cure. In cases of P vivax and Povale malaria, which do form hypnozoites, radical cure requires therapy with both a blood schizonticide and a tissue schizonticide.
Recurrence of malaria infections after treatment is due either to recrudescence or to relapse. Recrudescence occurs when the claret schizonticide does non eliminate all parasites from the claret stream, either because the dose was inadequate or because the parasite is resistant to the drug. Relapse occurs in P vivax and P ovale infections subsequently the delayed development of liver- stage parasites that have not been treated adequately with a tissue schizonticide.
Resistance of malaria parasites to antimalarials may be consummate or relative; relative resistance can exist overcome by raising the dose of the antimalarial.
The choice of blood schizonticide depends upon the clinical status of the patient, infecting species and possibility of drug resistance. Parenteral therapy is reserved for patients unable to take medications by mouth and for those with complicated malaria.
Chloroquine-resistant P falciparum is widespread and currently exists in all malarious areas of the world except Mexico, Cardinal America, the Caribbean and parts of the Middle East. P falciparum resistant to multiple drugs is most prevalent in South.E. Asia simply is also nowadays in Africa and Brazil. Chloroquine-resistant P vivax is prevalent on the island of New Guinea. Primaquine-resistant P vivax is nigh prevalent in S.Due east. Asia and Oceania and is reported from other areas. Drug resistance has non been reported for P ovale or P malariae.
If ever in doubt as to infecting species or presence of resistance, clinicians should assume the infection to be chloroquine-resistant P falciparum. Such therapy will cover all malaria species, although side effects may exist more mutual.
The response to antimalarial therapy is monitored both clinically and by examining repeated blood films. Blood smears should be connected daily in all malaria patients until parasites are no longer detected. In severe or complicated malaria, parasitemia should be evaluated twice daily. Parasitemia should decrease by 75% and clinical status improve within 48 hour after initiating therapy. If non, drug resistance, inadequate drug levels or the presence of clinical complications should exist suspected.
Treatment of Specific Infections
Uncomplicated, chloroquine-sensitive infections
All patients with simple P malariae, P ovale,and P vivax and P falciparum from chloroquine sensitive areas (see above and Fig 83-6) should be treated with oral chloroquine. The drug is highly effective, well tolerated and inexpensive.
Unproblematic, chloroquine-resistant P falciparum
Therapy of chloroquine-resistant P falciparum is complicated and depends primarily on surface area of illness acquisition. Patients with uncomplicated affliction caused in areas of chloroquine resistance can be treated with 1 of several regimens effective against chloroquine-resistant parasites. In the The states, ii regimens are used primarily: (1) mefloquine alone, or (two) quinine, plus doxycycline or pyrimethamine/sulfadoxine (FansidarR). Other effective drugs include halofantrine, artemisinin (qinghaosu) derivatives, and clindamycin. Halofantrine and artemisinin are used widely overseas but are non currently available in the U.Southward.
Uncomplicated, chloroquine-resistant P vivax
Chloroquine-resistant P vivax is highly prevalent on the island of New Guinea (Papua New Guinea and Irian Jaya, Indonesia) and may be present elsewhere. Recent studies in Indonesia take shown halofantrine, and chloroquine plus primaquine to be highly effective against these resistant strains. Although not specifically tested, the above regimens for chloroquine-resistant P falciparum should also be effective.
Complicated infections
Astringent or complicated malaria is a medical emergency. It is acquired almost exclusively by P. falciparum. Patients with complicated malaria (run across Clinical Manifestations above) should be treated with intravenous antimalarials and in an intensive intendance unit whenever possible. The drugs of option are intravenous quinidine or quinine (IV quinine no longer available in the U.S.). Patients on these regimens must be observed closely for signs of hypotension or myocardial conduction abnormalities. Therapeutic plasma levels are 5 to fifteen μg/ml for quinine and 5 to 10 μg/ml for quinidine. Oral quinine, plus doxycycline or FansidarR, is substituted every bit soon equally in that location is clinical comeback. If acquired in an surface area of chloroquine-sensitive parasites, parenteral chloroquine may too be given. Artemisinin compounds show promise for therapy of astringent malaria because they decrease parasitemia faster than all other antimalarials.
Any complicated P malariae, P vivax, or P ovale infection should be treated in the same way as a complicated P falciparum infection, since mixed infections are common.
Radical cure of P vivax and P ovale infections
For infections due to P vivax or P ovale, primaquine should be given after therapy of the blood-stage infection to eradicate hypnozoites of these species and prevent relapses. P vivax with decreased sensitivity to primaquine is prevalent in SE Asia and Oceania, where up to 30% of cases relapse later on the standard regimen of xv mg/twenty-four hours primaquine base of operations for fourteen days, and is reported from other areas. Resistance is usually relative and most initial failures respond to 30 mg/day for 14 days. Primaquine should be used with circumspection in persons who are G6PD deficient due to its potential to cause severe hemolysis.
Ancillary Therapy and Treatment of Complications
Supportive care and therapy of malaria complications may be as disquisitional as choosing the correct antimalarial. Clinicians should monitor patients for complications (see Clinical Manifestations) and treat them equally they occur.
Hyperthermia should exist treated with cooling blankets and antipyretics. Proper fluid direction is essential to forestall renal failure or pulmonary edema. If oliguric renal failure persists afterwards fluid status is corrected, the patient is treated like other patients in the oliguric stage of acute tubular necrosis. Pulmonary edema, which may present similar the adult respiratory distress syndrome (ARDS), is an uncommon simply frequently fatal complication of severe P falciparum infection. Information technology is treated by careful fluid direction and application of the principles used in treating ARDS. Transfusion of erythrocytes may be necessary for severe anemia. Seizures are frequent with cerebral malaria and should exist treated with standard anticonvulsants. Corticosteroids are of no benefit in the therapy of cerebral malaria. Hyperparasitemia may exist treated with exchange transfusion. Commutation transfusion is generally reserved for individuals with more than 15 pct parasitemia or more than 5 pct parasitemia with cerebral malaria or other severe manifestation. Plasma glucose levels should exist monitored regularly and hypoglycemia treated if it occurs. Aspiration pneumonia may occur when unconscious cerebral malaria patients suffer seizures and vomiting. Aspiration is prevented by controlling seizures and by attention to general airway management in the unconscious patient. Gram-negative bacteremia is a frequent accessory of severe P falciparum infection. Gram-negative organisms probably enter the circulation in areas of the bowel wall that are ischemic as a effect of microcirculatory obstruction by parasitized erythrocytes. Any patient who is not responding to antimalarial therapy every bit expected should exist investigated for bacteremia. Hypotension and stupor may complicate severe malaria. If these occur, treatable causes should be considered, including Gram-negative sepsis, gastrointestinal hemorrhage, hypovolemia, and splenic rupture. Splenic rupture is seen infrequently only is one of the few fatal complications of vivax malaria.
Recent studies have establish a potent clan between sustained lactic acidosis and poor outcome in severe malaria. Until further work defines the role of specific interventions (due east.g., sodium dichloroacetate and sodium bicarbonate) in reversing lactic acidosis in severe malaria, treatment must be aimed at the correction of defects in oxygenation and tissue perfusion and metabolic abnormalities such as hypoglycemia.
Special Conditions
Malaria during pregnancy presents a unique trouble. Pregnant women are at higher risk of developing astringent and fatal malaria. Hyperparasitemia, hypoglycemia and pulmonary edema are more mutual in pregnant women with P falciparum infections. Pregnant women should be treated promptly with advisable doses of antimalarials. Quinine does not appear to induce labor equally was once idea. Significant women with chloroquine-sensitive P vivax infections should be treated with chloroquine to eliminate the erythrocytic-stage infection and and so placed on weekly chloroquine to prevent relapse, as the safety of primaquine in pregnancy is non known.
Prevention of Malaria
Individuals with lilliputian or no previous exposure who develop malaria may rapidly progress to severe, oftentimes fatal disease. Near cases of malaria in Americans can be prevented past chemoprophylaxis and by avoiding the mosquito vector.
The female person Anopheles mosquito feeds from dusk until dawn. During these hours, individuals should avoid contact with the mosquito by wearing protective clothing, using an insect repellent containing N,N-diethyl-l-m-toluamide (DEET), staying in screened areas and spraying these areas with pyrethrumcontaining insecticides, and past sleeping under insecticide-impregnated bednets.
Travelers to endemic areas should be advised not simply on avoiding the musquito vector but as well on chemoprophylaxis. It must be emphasized that chemoprophylaxis is not one hundred per centum constructive; regardless of prophylaxis, malaria must be considered in the differential diagnosis of any febrile affliction in an private who has been in an area owned for malaria within the terminal 2–3 years.
Chemoprophylaxis is designed to kill the parasite later information technology has gained access to the body but before it leads to the rupture of host RBCs, which causes the symptoms of malaria. Drugs may reach this by attacking the parasite in either the liver or the blood. Causal chemoprophylaxis refers to killing the parasite in the liver earlier it gains access to the claret. Suppressive chemoprophylaxis is achieved by drugs which attack asexual parasites in the blood. About antimalarial drugs assault parasites in the blood and are therefore suppressive chemoprophylactics. Primaquine is the simply antimalarial drug currently available which reliably kills liver stage organisms.
The pick of a chemoprophylactic regimen depends on several factors: the health of the individual (including factors such every bit pregnancy, age, and chronic illness); the take chances and types of malaria in the areas to be visited; and the presence of drug-resistant P falciparum.
Chloroquine is the recommended chemoprophylactic for those travelling to areas where plasmodia are still chloroquine sensitive (Mexico, Central America, Haiti, the Dominican Republic, and the Eye E). There are very few contraindications to chloroquine. Most travellers, even so, visit areas where there is chloroquine resistance and other drugs, generally with greater toxicity, must be used. For most of these travelers, mefloquine is the drug of option and doxycycline is every bit acceptable culling. All-encompassing mefloquine resistance makes doxycycline the drug of selection for those visiting the borders of Thailand. Chloroquine plus proguanil (proguanil is non available in the U.Due south.) is another possible regimen for chloroquine-resistant areas, but this regimen is much less constructive than mefloquine or doxycycline. Recent piece of work also suggests that primaquine, plainly acting against liver-stage organisms, is equally effective as mefloquine and doxycycline for chemoprophylaxis in areas of chloroquine resistance.
Prophylaxis with chloroquine or mefloquine should begin two weeks before entering the malarious surface area (to ensure tolerance to the drug and to provide adequate claret levels) and should continue throughout the stay in the area and for 4 weeks after leaving. Doxycycline should be started 1 to 2 days earlier travel to a malarious area and should exist taken daily during the stay in the expanse and for 4 weeks afterwards leaving. Taking the drugs after leaving the malarious area is referred to every bit terminal prophylaxis and is necessary to kill organisms which emerge from the liver afterward the person returns home. When there has been a meaning risk of exposure to P vivax or P ovale, primaquine should exist taken for xiv days after returning home to eliminate remaining liver stage parasites. Primaquine may be taken whatever fourth dimension during the 4 weeks in which the blood schizonticide is beingness taken. Run across Table 83-ane for drug dosages.
Table 83-i
Drugs Used For Chemoprophylaxis of Malaria.
Control in Populations
Control of malaria is hard and requires the sustained effort of many individuals from many disciplines (Fig. 83-8). It is much more easily achieved in some areas of the world than others. Control tin be extremely difficult in areas where the Anopheles vector is numerous, longlived, and feeds simply on humans.
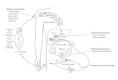
Figure 83-8
Strategies for prevention of malaria. (Adapted from Miller LH, Howard RJ, Carter R et al: Inquiry toward malaria vaccines. Scientific discipline 234:1350, 1986, with permission.)
Manual of malaria requires the presence of three factors: (one) malaria-infected humans conveying gametocytes that are infective to mosquitoes, (two) Anopheles mosquitoes that live long enough for the malaria parasites to develop within them to the infective sporozoite stage, and (three) infected mosquitoes that bite noninfected humans. Malaria command can be applied at each of these points: past treating man malaria infections and thereby reducing or eliminating the number of infected humans that mosquitoes feed on, by eliminating or reducing the numbers of Anopheles mosquitoes, by shortening the life span of mosquitoes to less than that required for the parasite to develop, or by providing alternative hosts for the mosquitoes to feed on.
At that place have been numerous efforts to reduce transmission by treating infected humans with drugs that render them noninfectious to mosquitoes. The success of these efforts is unclear.
Major efforts are under way to develop vaccines against malaria. Vaccines may be directed against any of the multiple stages of the organism's life cycle. Some vaccines effort to prevent or diminish illness in the individual by inducing immune responses against sporozoites, liver-stage parasites, or erythrocytic-stage parasites, or by preventing the release of cellular products idea to be involved in pathogenesis. Other vaccines endeavor to block manual to others by inducing antibodies or cytokines that attack gametocytes, or antibodies that preclude evolution of the extracellular stages within mosquitoes. Several vaccines are currently undergoing evaluation in clinical trials. Recently noteworthy is SPf66, a synthetic vaccine produced in Colombia which contains peptides from the organism'southward blood and sporozoite stages. Although initial results were promising, subsequent clinical trials accept reported protective efficacies of just about 30% against showtime clinical episodes of P falciparum. Farther evaluation is needed to define the role of this vaccine in reducing the morbidity and mortality from malaria in diverse settings.
A second approach in malaria control is to reduce transmission past eliminating mosquitoes--primarily by eliminating convenance places such as lagoons and swamps or by killing the larvae in these breeding places. This approach has been quite successful in some parts of the world, particularly in areas where malaria transmission is not intense. Transmission can also exist reduced by the apply of insecticide-impregnated bednets. Several studies have shown the effectiveness of these bednets in reducing the morbidity from malaria in areas of intense transmission.
Another approach is to treat dwellings with residuum insecticides, such as Dichloro-diphenyl-trichloroethane, that shorten the lifespan of mosquitoes, thereby reducing the chance that they volition live long plenty to transmit malaria. This arroyo has been quite successful in some parts of the globe, but has had meaning bug because of the development of mosquitoes resistant to insecticides.
In some areas of the world where malaria vectors prefer animals, such as cows, to humans, the introduction of these animals has reduced malaria manual.

Figure 83-7
Treatment of astute malaria. (Adapted from Miller LH, Howard RJ, Carter R et al. Research toward malaria vaccines. Science 234: 1350, 1986, with permission.)
References
-
Beadle C, Long GW, Weiss WR. et al. SL Diagnosis of malaria by detection of Plasmodium falciparum HRP-ii antigen with a rapid dipstick antigen-capture assay. Lancet. 1994;343:564. [PubMed: 7906328]
-
Bruce-Chwatt LJ: Essential Malariology, third ed. Edward Arnold, Boston, 1993.
-
Centers for Disease Control: Health Information for International Travel 1993. HHS Publication (CDC) 938280. Washington, DC, 1993 .
-
Proficient MF, Saul AJ,(eds.) Molecular Immunological Considerations in Malaria Vaccine Development. Boca Raton, CRC Printing: 1993 .
-
Hoffman SL. Diagnosis, Handling and Prevention of Malaria. Med Clin N Am. 1992;76:1327. [PubMed: 1405822]
-
Kozarsky PE, Lobel HO. Antimalarial agents: are we running out of options? Curr Opin Infect Dis. 1994;7:701.
-
Mendis KN, Carter R. Clinical Disease and Pathogenesis in Malaria. Parasitol Today. 1995;11:2–15. [PubMed: 15275352]
-
Tanner M, Teuscher T. Alonso PL. SPf66 - The starting time malaria vaccine. Parasitol Today. 1995;xi:10.
-
Warrell DA, Molyneux, Beales PF, eds. Severe and Complicated Malaria. Trans R Soc Trop Med Hyg 84 (Suppl two):1-65, 1990 .
walkerweepame1960.blogspot.com
Source: https://www.ncbi.nlm.nih.gov/books/NBK8584/
0 Response to "what phylum does plasmodium belong to? (use your textbook.)"
Post a Comment